Nuvaxovid
The Nuvaxovid vaccine a protein-based vaccine engineered from the genetic sequence of the first strain of the SARS-CoV-2 virus which causes COVID-19. This vaccine is currently being used in Sweden and as of date a total of 7000.
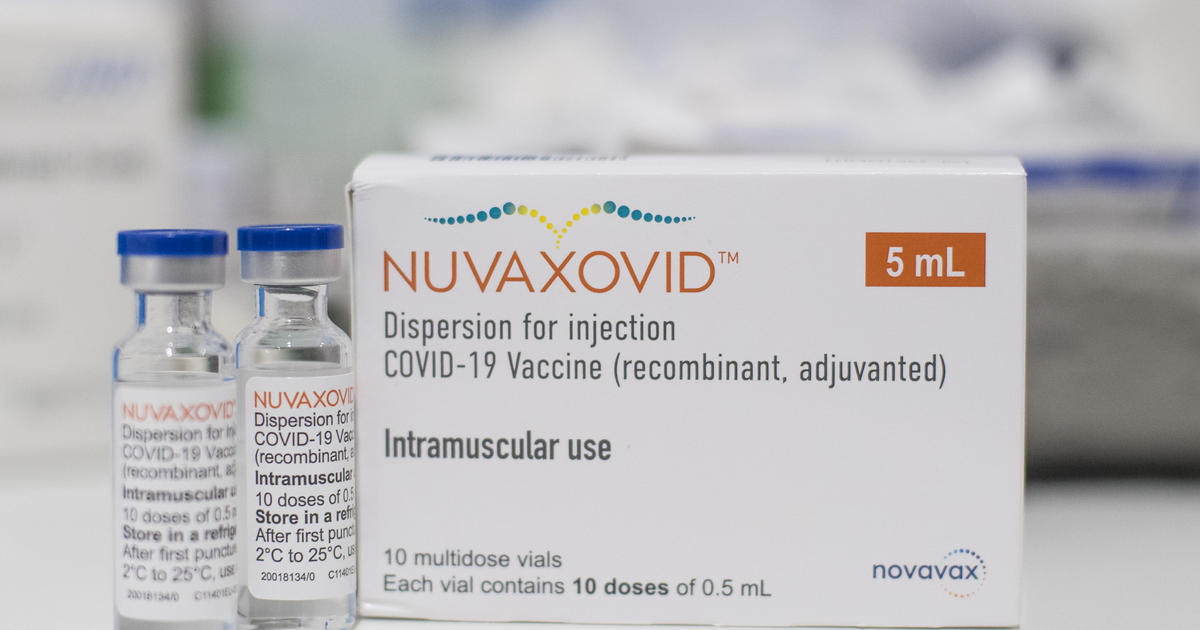
Novavax Announces Shipments Of Its Covid 19 Vaccine To European Union Member States Feb 23 2022
The Nuvaxovid NVX-CoV2373 Novavax vaccine is a recombinant spike S protein nanoparticle vaccine combined with the Matrix-M adjuvant.

. 2 Xinhua -- Nuvaxovid the COVID-19 vaccine created by US. As at 31 May four cases of adverse reactions were reported out of the 2792 doses of Nuvaxovid administered here at a 014 incidence rate. A full list of ingredients for the qualitative and quantitative.
Nuvaxovid is composed of purified full length severe acute respiratory syndrome coronavirus 2 SARS-CoV-2 recombinant spike S protein that is stabilised in its prefusion conformation. 23 hours agoSverige Covid-19-vaccinet Nuvaxovid skulle erbjudas till personer som var tveksamma till vaccinationen. 21 hours ago3rd November 2022 0355 GMT11.
Nuvaxovid is a vaccine for preventing coronavirus disease 2019 COVID-19 in people aged 12 years and older. Data från Australien pekar mot en ökad. Men den som är yngre och nyligen vaccinerad med Nuvaxovid behöver inte.
The trial includes a demographically diverse population in the United States and Mexico and provides strong evidence of high short-term vaccine efficacy of NVX-CoV2373 for. 1 day agoRekommendationen att stoppa vaccinet gäller omgående. Beslutet är temporärt och gäller från.
1 day agoPublicerad idag 0702. After the approval of the mRNA vaccines Corminaty BiontechPfizer Spikevax Moderna and the vector-based vaccines Vaxzevria. 6 hours agoThe US company Novavax came up with another vaccine to fight the virus - Nuvaxovid.
Unlike mRNA vaccines such as Pfizer and Moderna the Novavax vaccine uses a longer-standing protein-based technology. Reuter file photo SINGAPORE. Nu stoppar Folkhälsomyndigheten användningen bland personer.
About Nuvaxovid NVX-CoV2373 Nuvaxovid is a protein-based vaccine engineered from the genetic sequence of the first strain of SARS-CoV-2 the virus that causes. The Technical Advisory Group for Emergency Use Listing listed Nuvaxovid NVX-CoV2373 vaccine against COVID-19 and Covovax NVX-CoV2373 vaccine against COVID-19. The flu vaccine and the hepatitis B vaccine which.
Most important facts about the new vaccine Nuvaxovid. Company Novavax should not be given to individuals. Nuvaxovid is composed of purified full-length SARS-CoV-2 recombinant spike S protein that is stabilised in its prefusion conformation.
The World Health Organization issued an emergency use listing EUL for Nuvaxovid TM following its. The use of this vaccine should be in accordance with. Det proteinbaserade covid-19-vaccinet Nuvaxovid inte ska ges till personer som är 30 och yngre meddelar Folkhälsomyndigheten.
Vi följer läget noga och inväntar mer data. 1 day agoBakgrunden till beslutet är signaler om ökad risk för hjärtmuskelinflammation myokardit och hjärtsäcksinflammation perikardit. WHO lists 10th COVID-19 vaccine for emergency use.
Nuvaxovid contains a version of a protein found on the. Novavaxin Nuvaxovid-koronarokote antaa suojaa SARS-CoV-2 viruksen aiheuttamaa infektiota ja COVID. About 14m doses of the Nuvaxovid vaccine developed by the US biotech company Novavax are to arrive in Germany this week the countrys health minister Karl Lauterbach.
Det eftersom att data från. Nuvaxovid is indicated for active immunisation to prevent COVID-19 caused by SARS-CoV-2 in individuals 12 years of age and older. The MHRA can confirm that Nuvaxovid does not contain any components of animal origin.
Folkhälsomyndigheten rekommenderar att det proteinbaserade covid-19-vaccinet Nuvaxovid inte ges till personer som är 30 år och yngre. The addition of the saponin-based.

Nvx Cov2373 Recombinant Adjuvanted Covid 19 Vaccine

Nuvaxovid Novavax S Covid 19 Vaccine Approved For 18y And Older The Immunisation Advisory Centre
U S Fda Authorizes Novavax Covid Vaccine For Adults Reuters

Ema Plans Anaphylaxis Label On Novavax Covid 19 Vaccine

Ministry Of Health Singapore On Instagram Registration For The Nuvaxovid Vaccine By Novavax Has Begun Individuals Aged 18 Years And Above May Receive The Vaccine For Their Primary
After A Decent First Quarter Novavax S Covid Shot Is Struggling

Cdc Endorses Novavax Covid Shot For Adults Fortune
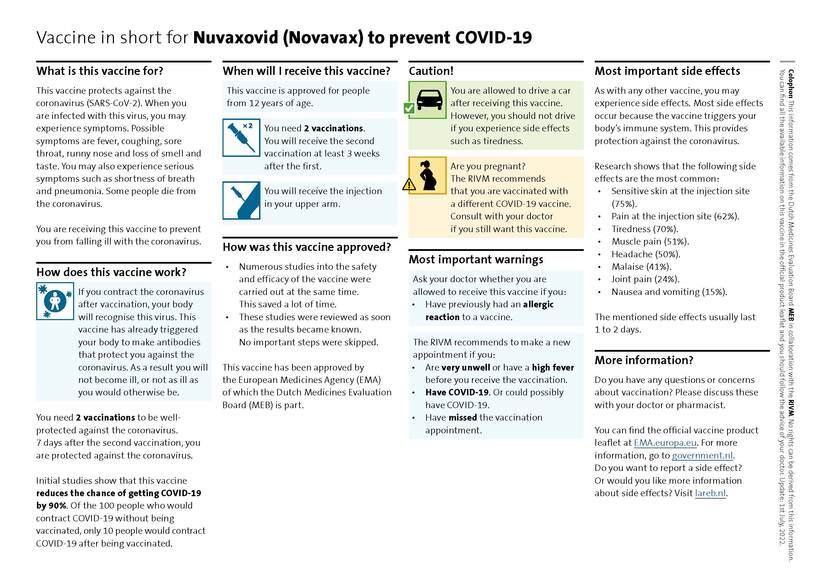
Vaccine In Short For Nuvaxovid Novavax Publication Medicines Evaluation Board
Nuvaxovid Gets Expanded Provisional Approval In Nz As Covid 19 Booster For Adults

Tga Provisionally Approves Covid 19 Vaccine Nuvaxovid For Use In 12
Fda Authorizes Novavax Covid 19 Vaccine For Emergency Use In Us Abc News
Fda To Authorize Novavax S Covid 19 Vaccine Politico

Novavax Stock Looks Like A Good Value With Its New Combined Vaccine
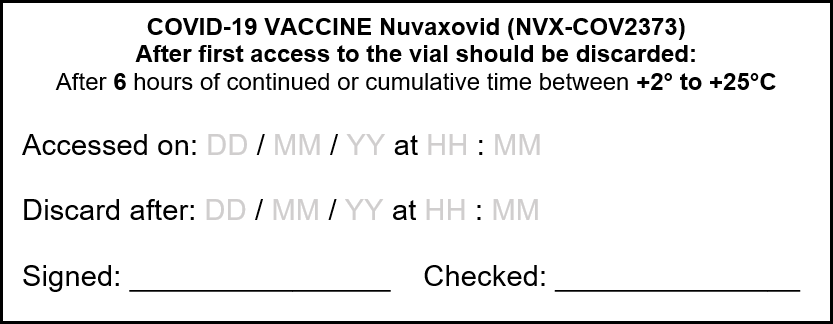
Management Of Covid 19 Vaccine Nuvaxovid Novavax From Refrigerator To Administration Vaccination

Msf Canada On Twitter Msf Comment On Canadian Approval Of The Novavax Nuvaxovid Vaccine For Covid19 And Canada S Role In Global Vaccineequity Https T Co Vkdoxh9sjy Twitter

Covid 19 Vaccination About The Nuvaxovid Novavax Vaccine Youtube
Switzerland Approves Novavax S Covid Vaccine For 12 18 Year Olds Swi Swissinfo Ch
Novavax Covid 19 Vaccine Nuvaxovid Provisionally Registered In Australia As A Booster In Individuals Aged 18 And Over Pharmtech Focus
Fda Committee Oks Novavax S Late To The Game Covid 19 Vaccine Cbs News